The PROTAC Gold Rush
The development of targeted protein degradation is so strong that in July 2021, Pfizer made an upfront payment of $650 million plus a $350 million equity investment to Arvinas to co-develop PROTAC, currently in Phase 2 trials.
In August 2021, Bayer acquired drug discovery company Vividion Therapeutics for $1.5 billion upfront and $500 million in potential milestone payments. Kymera announced a $2.15 billion partnership with Sanofi and then went public a month later, becoming the third PROTAC company to go public. There are now four publicly traded companies with a cumulative market value of about $11 billion. The tantalizing prospect of removing small molecules that target disease proteins from cells has spawned at least ten other biotechnologies, and at least six companies have brought Protac-like molecules into clinical trials (Figure 1).
The development of targeted protein degradation is so strong that in July 2021, Pfizer made an upfront payment of $650 million plus a $350 million equity investment to Arvinas to co-develop PROTAC, currently in Phase 2 trials. In August 2021, Bayer acquired drug discovery company Vividion Therapeutics for $1.5 billion upfront and $500 million in potential milestone payments. Kymera announced a $2.15 billion partnership with Sanofi and then went public a month later, becoming the third PROTAC company to go public.
There are now four publicly traded companies with a cumulative market value of about $11 billion. The tantalizing prospect of removing small molecules that target disease proteins from cells has spawned at least ten other biotechnologies, and at least six companies have brought Protac-like molecules into clinical trials (Figure 1).
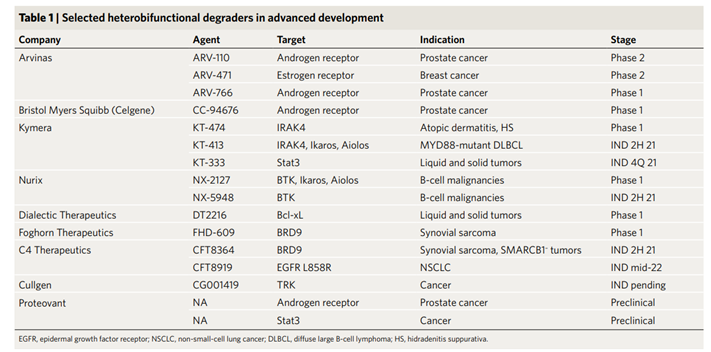
Figure 1 Information about companies that have developed PROTAC molecules and are already in clinical studies and their molecules
The potential to selectively degrade target proteins by utilizing the cell's own processing system is enormous. PROTACs It can degrade disease-causing proteins that could not be used as medicine and overcome traditional small-molecule resistance. But whether they will become a major new drug model is uncertain.
With the development of PROTACs, some limitations began to emerge, they were more difficult to develop than traditional small molecules, their toxicology was uncertain, and the side effects were largely unknown. "The next few years will be critical," says Nathanael, an organic chemist at Stanford University and a co-founder of C4 therapy. "The success of clinical trials will prompt significant investment in a field that has the potential to become, like biologics, a universal model for drug discovery. "Gray said.
1. The booming trend
PROTAC, or protein-targeting chimeras, degrades target proteins by utilizing the cell's own ubiquitin proteasome system (UPS). The small molecule is connected by the ligand of ubiquitin ligase, the ligand of the target protein, and linker, which triggers the transfer of the ubiquitin chain and labels and degrades the target protein by making the ubiquitin ligase contact the target protein (Figure 2). PROTACs was inspired by the concept of induced proximity in chemical biology, whereby certain small molecules in nature can work by bringing two proteins into close contact.
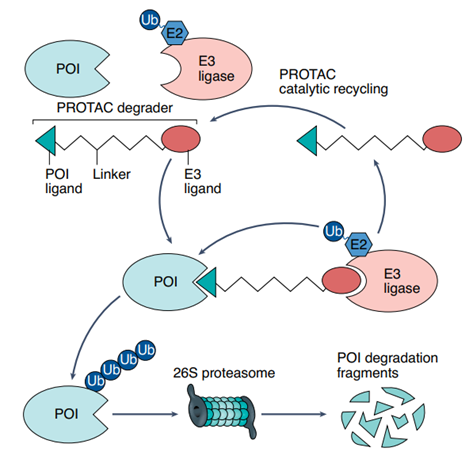
FIG. 2 Principle of PROTAC
Yale University chemical biologist Craig Crews and California Institute of Technology biochemist Ray Crews Deshaies described the first functional PROTAC in 2001. By allowing the target protein MetAP-2 to be absorbed by Skp1 -- Cullin -- F box is recognized and degraded. As the E3 ligand in PROTAC is a large polypeptide, its physical and chemical properties are not good and it has no practical commercial value (Figure 3).
It was not until 2015 that Craig Crews and colleagues reported the first nanomole activity of a small PROTAC molecule degrading ERRα and PIPK2 (Figure 4). And then in Dana Jay Bradner, an oncologist at the Farber Cancer Institute, and his team have designed a highly effective, highly selective CRBn-recruiting BRD4 depressant.
Yale University chemical biologist Craig Crews and California Institute of Technology biochemist Ray Crews Deshaies described the first functional PROTAC in 2001. By allowing the target protein MetAP-2 to be absorbed by Skp1 -- Cullin -- F box is recognized and degraded. As the E3 ligand in PROTAC is a large polypeptide, its physical and chemical properties are not good and it has no practical commercial value (Figure 3). Until 2015, Craig Crews and colleagues reported the first small PROTAC molecule with nanomole activity to degrade ERRα and PIPK2 (Figure 4). Followed by Dana Farber Cancer Institute oncologist Jay Bradner and his team have designed a highly effective, highly selective CRBn-recruiting BRD4 depressant.
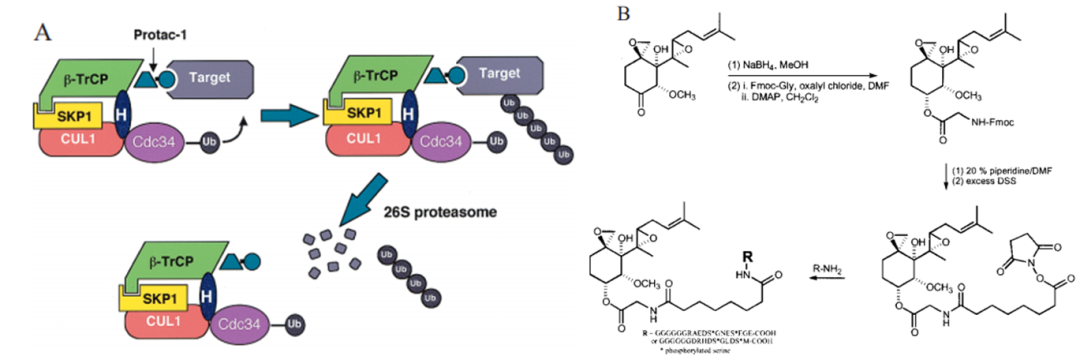
Figure 3. Structure and mechanism of action of the first PROTAC molecule
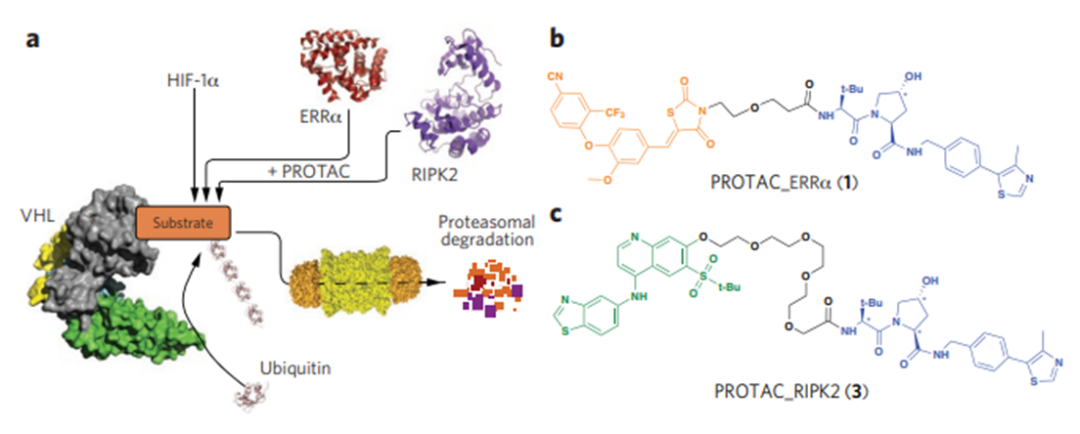
Figure 4 PROTAC molecule with activity at nM level
2. Difficulties abound
Although PROTAC molecule has many advantages, there are many difficulties in its development. Compared with traditional small-molecule inhibitors, they tend to have higher molecular weight (0.7-1.1kDa), more hydrogen bond donors, and larger polar surface area, resulting in poor membrane permeability, low oral availability, and relatively difficult synthesis. In addition, although there are more than 600 E3 ubiquitin ligases in cells, they are now mainly dependent on VHL and CRBN, so resistance is also possible. The design of the drug may also affect the conformation of the ternary complex and thus its degradation efficiency. So while many companies have rationalized to a certain extent PROTAC optimizes the process, but it is still largely empirical and more difficult to develop than conventional small molecule inhibitors.
3. Dose overload
Because PROTAC degradation is "event driven" rather than "occupation driven" and therefore requires only instantaneous binding to the target protein before the drug dissociates and moves on to the next target protein, PROTAC has the theoretical advantage of low doses. In the preclinical, very low The PROTAC dose significantly reduced protein levels, while the phase 2 dose of Arvinas' androgen receptor depressant ARV-110 was 420 Mg. The reason for the high dose is the low permeability and solubility of the compound. It is not difficult to see that oral bioavailability is a bottleneck in PROTAC development. Although VHL-based PROTAC It's easier to synthesize, and most companies are using cereblon instead of von Hippel-Lindau (VHL) E3 ligase to study oral depressants because of cereblon Ligands have better "drug-like" properties -- lower molecular weight, fewer hydrogen bond donors, and fewer rotatable bonds (Figure 5).
But it's totally dependent on cereblon Clinical application may be limited because other ligases may be more active or expressed in some tissues. Because PROTAC degradation is "event driven" rather than "occupation driven" and therefore requires only instantaneous binding to the target protein before the drug dissociates and moves on to the next target protein, PROTAC has the theoretical advantage of low doses. In the preclinical, very low The PROTAC dose significantly reduced protein levels, while the phase 2 dose of Arvinas' androgen receptor depressant ARV-110 was 420 Mg. The reason for the high dose is the low permeability and solubility of the compound. It is not difficult to see that oral bioavailability is a bottleneck in PROTAC development.
Although VHL-based PROTAC is easier to synthesize, most companies are using cereblon instead of von Hippel-Lindau (VHL) E3 Ligase to study oral antidotes because cereblon ligand has better "drug like" properties -- lower molecular weight, fewer hydrogen bond donors, and fewer rotatable bonds (Figure 5)[3]. But totally dependent cereblon may limit clinical use because other ligases may be more active or expressed in some tissues.
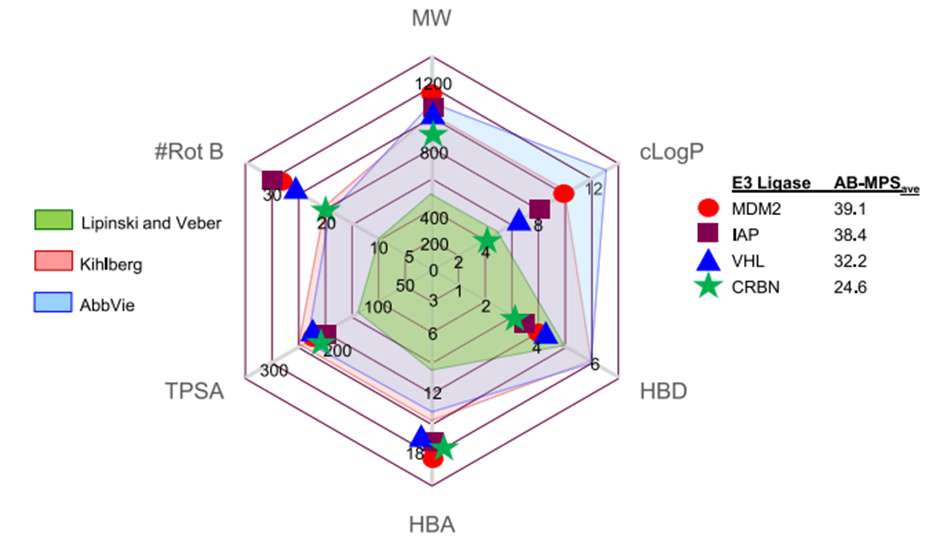
Figure 5. Physicochemical properties of common E3 ubiquitin ligand
4. Molecular glue
Given the dosing and oral availability challenges of PROTAC, some companies are focusing on another depressant: molecular glue. These are only combined with E3 The "univalent" small molecule of the ligase, rather than the bivalent that binds the ligase to the target PROTAC. Compared with PROTAC, molecular glue has the following advantages: small molecular weight, better physical and chemical properties, easier to develop drugs and does not require binding pockets on the target protein, while the disadvantage of glue is that it is difficult to degrade specific disease-related proteins. Molecular glues are generally discovered by working backwards, by screening E3 binds molecules to obtain the desired phenotype or performs proteomic screening, and then finds out which proteins they degrade.
5. Potential side effects
Toxicology is another bottleneck in the development of PROTAC, with potential for both target and off-target side effects. Target side effects refer to the toxicity of degrading proteins compared to inhibiting protein activity. Of course, completely removing a protein may also be more effective than just inhibiting its activity, so companies need large animal models to assess the toxicity of small molecules. PROTAC Warheads are usually very specific to their target, and active ternary complexes are generally a very selective depressant, but the possibility of potential misses cannot be ruled out, and the missed target will lead to protein degradation, resulting in its effects on the off-target for a long time.
6. Summary and outlook
In general, PROTAC provides great potential for the development of non-patent proteins. Although there are certain limitations in its molecular design, it has brought light to the treatment of human diseases. It is expected that PROTAC molecules can break through obstacles in clinical practice and bring more options for clinical drugs as soon as possible.


