Generics also have a spring in their step
According to the 2021 Drug Review Report, there were 908 registration applications for consistency evaluation of quality and efficacy of generic drugs (hereinafter referred to as consistency evaluation applications) in 2021.
The 908 conformance evaluation applications involved 331 drugs, compared to 219 in 2020, an increase of 33.8 percent over the previous year.
In other words, in 2021, an average of 2.7 pharmaceutical companies per drug passed the conformance evaluation. In practice, however, some drugs contain multiple sizes, such as amoxicillin capsules containing 0.125g, 0.25g and 0.5g, increasing the number of drugs and dragging down the average number of approved companies per drug.
Still, many have more than five approved companies.
The largest number of approved companies was omeprazole sodium for injection (40mg), which reached 23, amoxicillin capsule (0.25g) and ceftriaxone sodium for injection (1.0g), which also reached 18, fully demonstrating the fierce competition. Among them, omeprazole sodium for injection has been included in the seventh batch of national collection, barefoot enterprises are rubbing their hands, head enterprises will not be willing to hand over.
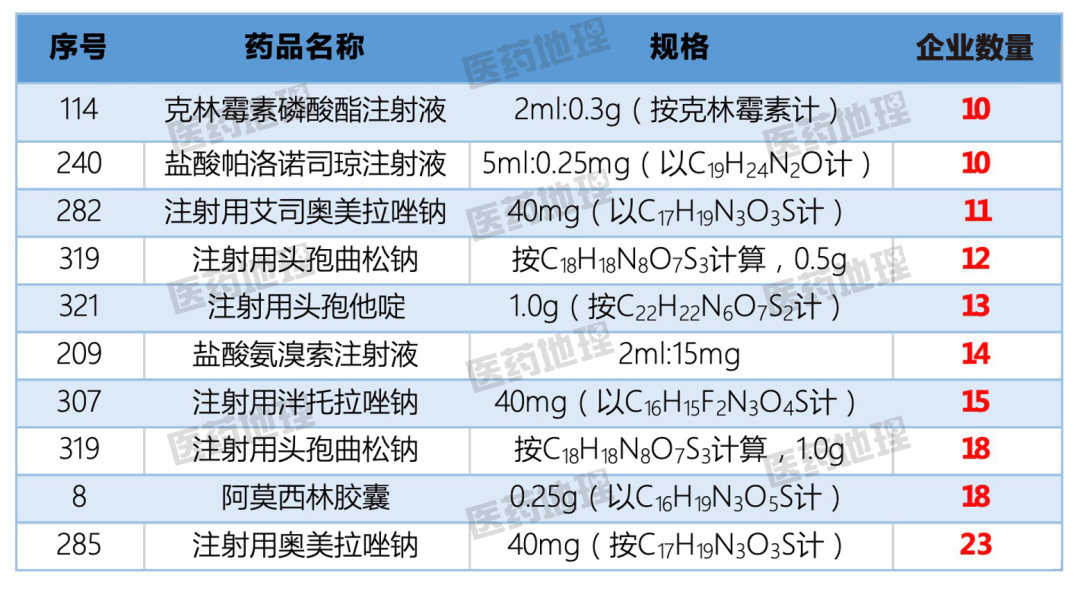
Figure 1: Consistency evaluation varieties with more than 10 approved enterprises in 2021
It can be predicted that with the continuous promotion of consistent evaluation, the competition of generic drugs will become more and more fierce, and corporate profits will be compressed. At that time, the generic drug enterprise is facing exit or transformation of these two roads? We'd rather know,Is there any chance that drug companies will win if they remain focused on generics?
Intensive compulsory courses
In addition to the increasingly severe competition, the collective impact on generic drug companies is more direct.
According to the medical price index monitoring conducted by the National Healthcare Administration, the price of chemical drugs dropped by about 7 percent year-on-year in 2019 and 2020. As more and more drugs are included and will be included in the collection, generics companies will continue to see revenues and profits squeezed, especially for those who have lost the majority of the market.
Acarbose sugar is one of the top three varieties in East China with annual sales of more than 3 billion yuan. In the second batch of state-organized centralized drug procurement, Acarbose sugar in Central America and East China lost its bid, which directly led to a 60% decline in revenue of public hospitals in 2020. This decline continued until 2021. According to the 2021 semi-annual report, the net profit of East China Pharmaceutical declined by 24.89%.
Collective purchasing is mainly carried out for generic drugs, while most domestic pharmaceutical companies rely on generic drugs for their main business and cash flow. Therefore, how to reduce the impact of collective purchasing on pharmaceutical companies has become a required course for pharmaceutical companies at present. Some enterprises begin to transform to innovation, such as Big Brother Hengrui, but it is undeniable that there is only one Hengrui in China. More small and medium-sized pharmaceutical enterprises have neither enough cash flow, nor corresponding talent reserve, nor the motivation and willingness to innovate, nor the courage to turn around, and finally choose to fight to the end in the generic drug market.
In this case,It is very important to choose the right generic drugs and find a differentiated competition way in the generic drugs.In fact, companies that only make generic drugs are still worthy of respect. Let's not talk about the big and empty focus and persistence. From the perspective of clinical application, it is cheap generic drugs rather than innovative drugs that meet the more common and basic needs of diagnosis and treatment.
The paradox of differentiated competition and market size
How to achieve differentiated competition? Obviously, the more competition there is, the less differentiation there is, unlike an innovative drug that can expand into a new indication,Generic drugs are essentially "metoo" non-inferior drugs, so it is better to choose products with relatively few competitors to carry out consistency evaluation.
But it's a back and forth game. In fact, the more competitive the varieties are, the larger the market scale is. The less competitive varieties tend to have a smaller market. The varieties without consistent evaluation either have no market or have technical difficulties in the evaluation process, so no one is willing to do it or dare to do it.
And that,Differentiated competitive strategies also vary with the size of the firm.For large pharmaceutical enterprises, capital and technology are dominant, so consistency evaluation of large varieties can be prioritized, and as many potential varieties can be completed as possible, so as to accumulate advantages in the speed and breadth of collection. Small and medium-sized pharmaceutical companies also have their own advantages, that is, "barefoot are not afraid to wear shoes." Collective purchase makes everyone stand at the same starting line, and the market base and sales team all go to zero. Small and medium-sized pharmaceutical companies are willing to lower the price than large pharmaceutical companies as long as they have completed the consistency evaluation for a certain large generic drug, because small pharmaceutical companies can easily realize the "price for volume", after all, the previous volume may be zero.
But then again,Small and medium-sized companies have won the market, but not necessarily the market, because the supply of drugs is also a problem.In the past two years, a lot of supply incidents occurred. "The eldest son of the Republic" North China Pharmaceutical opened a bad supply pioneer. The overall supply rate of ibuprofen sustained release capsules is less than 20%, which is a relatively strong pharmaceutical enterprise. Huabei Pharmaceutical explained the reasons for the new policy and the epidemic, but that is the crux of the problem. Misjudgment in management is more likely to occur in small and medium-sized pharmaceutical enterprises. Once it happens, the anti-risk ability and rapid adjustment ability will drag their shoulders, which will ultimately affect the treatment of patients and cannot be delayed.
This is why it is generally believed in the industry that collectivization tends to produce the leading pharmaceutical companies, which will promote the re-integration of the industrial chain and eliminate the backward production capacity. For example, Japan, the "rock of the mountain" of generic drugs, has only more than 200 generic pharmaceutical companies in the whole country at present.In the future, pharmaceutical companies with cost advantages, technological advantages and scale advantages are more likely to win.
"Dapeng rides the wind in a day, soaring to 90,000 miles", differentiation of competition sounds good to say, but the specific operation, it really needs each pharmaceutical company to have a clear mind, ability and opportunity are indispensable. The most irrefutable thing I'm afraid is to think well before you do it. Just like the management master Drucker said, a successful company must have a successful strategy, but the successful strategy is based on a clear understanding of the reality under the accurate trend judgment.
Summary
Since the implementation of conformance evaluation and collection, it is evident that the term transformation and upgrading appears more and more frequently in the pharmaceutical industry. Often "innovation", "first imitation", "high-end preparation", so the author is curious, why did not focus on the transformation and upgrading of generic drugs itself? It feels like everyone is busy "transforming" rather than "upgrading." Perhaps this is also an illusion created by social media, where "turning" is bigger and more likely to attract attention.
I read an article a few days ago, which said that Trodo (the one selling yogurt) has been in the pharmaceutical business since 1961. Despite N years of mediocre performance, it insists on the research and development of anti-cancer drugs. It is important to participate in the free and easy, and it is the spirit of ingenuity that is devoted to research and development. Both generic drug consistency evaluation and innovative drug research and development in China have a relatively short time to start. People rush to the market in a rush and anxiety. Should we stop and think about what is right and what is worth sticking to at the crossroads of transformation?
In the next three to five years, we invite you to come back.


